What is zirconium and where is it used?

The oldest metal has unique properties that are in demand in various spheres of life. With the development of new production technologies, the popularity of zirconium is only increasing. Historical data, as well as the properties and composition of a chemical element, will help to unravel this riddle.


What it is?
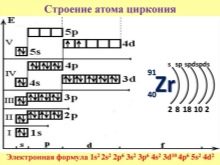
In the famous table of D.I. Mendeleev, the atomic number of zirconium corresponds to 40, its designation is Zr. Zirconium belongs to the 4th group, stands in the 5th period. The mass of the atom is 91.22 g / mol. The radius of the atom corresponds to 160 picometers. In the center is the nucleus, as well as neutrons and protons. There are 5 orbits around the atom, which contain 40 electrons. Natural material is present in the form of oxides, salts or silicates. Outwardly, the metal looks like steel. The composition of the metal element may include additional impurities, due to which different colors and shades are obtained.


Zirconium should be distinguished from zircon and cubic zirconium. The fact is that zirconium is a metal that resembles steel. Zircon is a natural stone that resembles gold. It contains zirconium atoms.
Cubic Zirconia is a man-made crystal that has a luster and a diamond-like appearance. It is produced using zirconium oxide at high temperatures.


Origin story
According to scientists, zirconium is the oldest chemical component, formed 3.5 billion years ago. Its traces have been found in the deepest layers of the earth's crust. In the process of studying the zircon mineral, a scientist from Germany Klaproth was able to isolate an insoluble concentrate, zirconium dioxide. This event took place in 1789.
The Swedish scientist Berzelius was able to isolate zirconium as a free element in 1824. A pure chemical element was obtained only in 1925. Dutch chemist Anton Edurard van Arkel managed to do this. The origin of the name of the chemical element itself is still unknown. There is an opinion that the roots should be found in the Arabic language. There is a point of view that the name came from the Persian language.


Basic properties
Metal with a silvery color has good ductility. Zirconium is resistant to corrosion, alkaline solutions, does not degrade in an acidic environment. It can be easily processed by rolling, forging or rolling. Outside, the material has an invisible coating that reliably protects against the effects of gases, steam, water. The element is resistant to the influence of high temperature indicators, ammonia, acids, alkalis.


In a powdery state, even at normal room temperature, a chemical element is explosive, it is highly flammable. It should be noted that the element is useful as an impurity. It is added to the composition of various alloys. This increases the strength characteristics, wear resistance of the resulting material. However, the addition of any impurities to a chemical element is unacceptable, since it significantly impairs its properties.
It should be noted that the chemical element has low strength, which can lead to chips, loss of aesthetic appearance. The level of strength is influenced by the amount of gas components. The greater their number, the lower the strength indicators.


Physical
- At a temperature of 20 C, the density of zirconium is 6.5 g / cm3.
- The strength is 175 MPa.
- The elasticity index is 96 MPa.
- The melting point is 1855 C.
- The boiling point is approximately 4350 C.
- Thermal conductivity is 300 K.
- Zirconium magnetizes when heated.
- In its pure form, the element is plastic. However, the addition of impurities in the form of nitrogen, oxygen, hydrogen, carbon makes the material brittle.
- Brinell hardness is 640-670 MN / m2. This indicator is greatly influenced by the presence of oxygen. The more it is, the more hardness. With a high hardness, zirconium cannot be processed by pressure. Interaction with oxygen is provoked by an increase in temperature indicators.
- Zirconium is stable in water at temperatures up to 300 C.
- Vickers hardness ranges from 600 to 1700 MPa.


Chemical
- Zirconium has the highest oxidation state, which is +4. A lower oxidation rate can be found in the metal in the presence of impurities in the form of chlorine and bromine. Oxidation occurs at temperatures of 200-400 C.
- When the element is heated to 250 C and above, hydrogen is absorbed, as a result of which hybrids with metallic properties are formed.
- Interaction with halogens promotes the formation of zirconium halides.
- Oxides are formed at temperatures of 500 C and above.
- Electronegativity is zero.
- The covalent radius is 145 pm.
It should be noted that physicochemical indicators are variable. They change depending on the presence of certain impurities.

Methods of obtaining
Zirconium is mined from ore concentrates. It can often be found in the form of oxides, silicates. In a pure chemical element, it does not occur in the earth's crust. In nature, you can find zircon, baddeleyite. Deposits of a chemical element are scattered all over the world. Large deposits are rare. A large number of ore deposits are found in Australia, Brazil, India, South Africa. V Russia is considered to be rich in ore reserves in the Murmansk, Tomsk, Tambov, Nizhny Novgorod regions. The first place in terms of the availability of zirconium reserves is occupied by the Kola Peninsula.
Often, in the ore, along with zirconium, hafnium is present, which has similar properties. Each element individually has valuable characteristics, but their combination is unacceptable, as it makes the natural material unsuitable for use. In order to separate one chemical element from another, a multi-stage purification system is used. This significantly increases the cost of zirconium production. In industry, zirconium dioxide concentrates are used, which are obtained by enriching ore.



There are various methods of metal recovery.
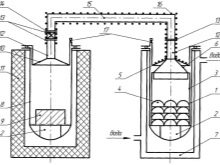
- Chloride. Krol's method is based on the chlorination of zirconium dioxide. Further, cleaning, restoration with the help of magnesium is carried out. The frequent use of the method is due to its relatively inexpensive cost. There are 2 ways of chlorination. The direct process is carried out at temperatures from 900 C to 1000 C. The second method consists in chlorination of the initial mixture at temperatures from 400 C to 900 C.
- Fluoride. The method involves sintering a concentrated mixture of zirconium with potassium at a temperature of 600 to 700 C. The resulting concentrate is alkalized and purified. Then electrolysis of the melt is carried out and the metal component is isolated.
- Alkaline. The method is designed to isolate zirconium dioxide. To obtain a metal element, a fluoride or chloride method is carried out. Through the alkalization process, the zirconium is converted into a soluble component. This is done by sintering, for example, with a mixture of calcium carbonate and calcium chloride at temperatures from 1000 to 1300 C. Then alkalinization, purification, hydrolysis and calcination are carried out. Thus, zirconium is obtained under laboratory conditions. From the end product, rods are formed, which are sent to production.


Species overview
Depending on the field of application, the chemical element can be presented as a hard alloy with a silvery tint or in the form of a blue powder. Gems synthesized from a chemical element can be of different shades. The description of the color, its intensity depends on the amount and type of impurities, for example, cerium, titanium, erbium, chromium. Also, zirconium silicate, which is part of zircon, is represented by the following varieties.
- Hyacinth. Has a brown, red or pink tint.
- Matarskiy diamond. The transparent stone has no color, it is mined on the island of Matara.
- Jargon. Differs in the presence of a straw, golden hue.
- Starlit. A transparent stone with a blue tint.
- Malacon. Has a dark brown color.






Applications
Depending on the type, state of a chemical element, its use is widespread in various spheres of life.
- The metal is used for industrial purposes, in jewelry, in everyday life.
- In industry, zircon, zirconium silicate, zirconium dioxide, baddeleyite are often used.
- In the metallurgical industry, the chemical element is used for alloying steels. It is added to alloys to improve quality. Thanks to zirconium, the strength increases, and the cutting process is facilitated.
- In the manufacture of pyrotechnics, zirconium is used in the form of a powder. When the mixture burns, there is no smoke, therefore it is used to create fireworks, salutes.
- In the chemical industry, zirconium is used in the manufacture of wear-resistant ceramics.
- The metal is widely used in the production of military equipment, for example, for the manufacture of distance bombs, bullets, rockets with lighting. Metal alloys are an integral part of nuclear reactor designs.
- The production of enamel, ceramics, glaze is also not complete without the participation of zirconium. In this case, zirconium oxide is used. It does not darken, has a presentable appearance, and improves product quality.
- The metal is used in leather tanning. In this case, compounds with sulfate are used.
- In the engineering industry, metal is used in the manufacture of pumps, shut-off valves.
- Unlike lead aprons, even a thin sheet of metal is much more effective in protecting against the penetration of X-rays.
- Due to the healing properties of the metal, it is used in various fields of medicine.
- Zirconium components do not cause an allergic reaction, rejection, wear resistance, strength, plasticity. They are used in traumatology to treat various fractures.
- The widespread use of zirconium dioxide in dentistry is due to the absence of an irritating factor when exposed to soft tissues or bone structure. Tools, dental implants, crowns, staples, plates, clamps, sutures are made of metal.
- Jewelry made of zirconium dioxide and cubic zirconium is famous not only for its exquisite appearance, but also for its beneficial effect on the entire body.
- The constant wearing of bracelets or belts has a healing effect on skin diseases, for example, eczema, dermatitis, psoriasis. Metal normalizes blood pressure, energizes, relieves insomnia. Zirconium helps to improve the condition in diseases of the musculoskeletal system.
- Also, the metal has a bactericidal effect. It is known for its ability to quickly heal wounds. If you put on zirconium earrings right after piercing your earlobes, healing will be faster.
- There is an opinion that the chemical element relieves pain, has a beneficial effect on the cardiovascular system, improves breathing, fights intestinal infections, viruses, and has an anti-cancer effect.
- The chemical element is actively used in lithotherapy in the treatment of diseases of the endocrine system.



There is an opinion that, depending on the color scale, there is a different effect on the body.
- For colds, it is recommended to use black metal.
- If you have a poor appetite, an element with a red tint will help.
- Brown is used to cleanse the body.
- Transparent minerals or blue stones help restore metabolism.

Tableware is made of zirconium, for example, mugs, spoons, glasses and other utensils. Also, the metal is used in optics, which are designed to operate under extreme conditions, for example, at high temperatures or sharp changes in it. Cubic zirconia have a large angle of refraction, this is used in the manufacture of lenses. Separately, mention should be made of the use of metal in jewelry. In ancient times, zircon was called an "imperfect diamond" because after being cut, their shine was more faded than natural stones.
It should be noted that small stones are used for jewelry, since they are safer in terms of radiation radiation. They are weakly colored and have little transparency. Large stones are characterized by excessive radioactivity. They are opaque and brightly colored. Such stones cannot be stored at home, cut or transported. Zirconium is used in the manufacture of rings, earrings, tiaras, pendants, pendants. Often make metal inserts into products, spraying.
There are uncoated jewelry, products with silver, cubic zirconia, and one-piece designs. They can be worn both for festive events and worn every day.



Proper care and storage will keep the jewelry intact. Cleaning is recommended with a cloth soaked in soapy water. Then the product is washed under water, wiped dry, polished with flannel. Stones are best kept separate from other jewelry. This will avoid mechanical damage.
In order not to be mistaken in the authenticity of the product, you should pay attention to the following points.
- Refraction structure of light rays. It should be multifaceted.
- The presence of a metallic luster. It is characterized by its uniformity.
- The presence of yellowish blotches.


Interesting Facts
In medieval times, Spanish jewelers made unique jewelry from zircon, which was highly radioactive. In appearance, they very much resembled precious stones. Wearing such jewelry was extremely dangerous. Even in ancient times, it was noted that long-term wearing of large dark-colored stones led to the imminent death of their owner. The hyacinth was named after the flower. According to mythology, Apollo grew a flower from the blood of a young man named Hyacinth, whom Apollo loved very much.
Due to the brilliance, the presence of different colors, the stone could be easily confused with diamond, sapphire, tourmaline or topaz. This was often used by fraudsters, passing off jewelry as jewelry. The inhabitants of Asia idolized the stone and endowed it with magical properties. For them, he was a talisman. Amulets and pendants were made from it.
The ancient sages believed that the stone endows the gift of clairvoyance. It was believed that the zirconium amulet protects against evil, envious people, promotes mental development.

In India, the stone was endowed with the ability to control the sun, the moon. It was believed that the talisman helps scientists, businessmen, wanderers, and also lovers in business. Astrologers also paid attention to the unique properties of the stone. In their opinion, zirconium is suitable for Aries, Capricorns, Aquarius. Its wearing should be limited, or even avoided altogether by Taurus, Libra, Sagittarius, Cancer.
So, yellow or blue minerals with a metallic glow are ideal for Aquarius. Wearing the product contributes to the development of intuition, analytical skills, and the emergence of exquisite taste. But for Capricorns, a blue-colored stone is suitable, which is recommended to be worn on the left side. This enhances the magical connection. Straw or red stones are more suitable for Aries. Wearing the product contributes to the development of magical abilities, attentiveness, caution.










