Nickel silver: what kind of metal is it and how to distinguish it from cupronickel?

On sale you can find a variety of dishes or jewelry made of metal that resembles silver. Many do not even suspect that they are made of nickel silver.

What it is?
Nickel silver that translated from German into Russian means "new silver"is not a specific metal. It is a three-component alloy based on copper, zinc and nickel. The percentage of these metals can be very different. The main element is copper, it can contain up to 60%. Nickel content is from 5 to 35%, zinc - from 13 to 45%.
The percentage of these metals determines the color of nickel silver, and it can be bluish or greenish in color. Its constituent components give it certain qualities. Nickel provides nickel silver with a silver tint, increases its resistance to corrosion, enhances chemical resistance: the alloy has no ability to dissolve in organic acids.

Copper adds qualities such as ductility and pliability, which improves its forgeability. Good plastic properties allow both hot and cold working.
Zinc presence provides high resistance to electric current. Also, the zinc content makes it cheaper compared to cupronickel, with which nickel silver has a similar appearance and some mechanical properties.


Nickel silver is distinguished by its high density, strength and elasticity during deformation, it lends itself well to polishing and retains a shiny surface for a long time.
History of origin
Nickel silver appeared thanks to cupronickel. This alloy was invented in China in the 8th century BC. Cupronickel had unique characteristics, became a replacement for silver, but was cheaper than the precious metal. Therefore, the Chinese scientists kept its formula a secret. Later, cupronickel products spread to European countries.
They became so popular that European metallurgists tried to discover its formula in order to independently produce this alloy. Soon, according to the assumption of the Europeans, they determined its composition. Scientists mistakenly believed that cupronickel contains copper, zinc and nickel. However, the percentage of the components remained undisclosed.
Only in the 19th century, German scientists obtained an alloy very similar to silver. But it was not cupronickel. A new metal with a lower cost in comparison with cupronickel and silver was named nickel silver... The resulting metal had a higher strength and stability: the process of its decomposition is observed only in hydrochloric and sulfuric acids heated to boiling.

The new alloy gained wide popularity and began to be used in the production of tableware, watches and jewelry.
Alloy composition options
Nickel silver is produced in accordance with GOST 5187-2003, which determines the chemical composition, all its mechanical characteristics. It has alphabetic and numerical markings: MSC and MSCS. The letters respectively designate copper, nickel, zinc, lead. The digital marking indicates the average permissible percentage of elements, and the amount of copper is not indicated: it accounts for the remainder. Since the percentage can be very different, there are about 50 brands of nickel silver.


The following brands are most commonly used in industry.
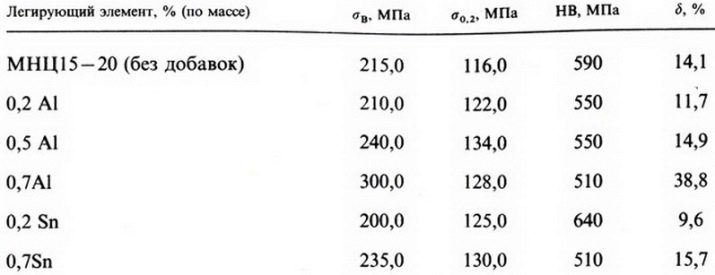
- MSC 15-20. This grade can contain nickel in the range of 13.5-16.5%, zinc - 18-22%, the rest is copper. It is also allowed to include additives such as antimony, silicon and others. Their share is insignificant and does not exceed 2%.
- MSCS 16-29-1.8. In this version of nickel silver, in addition to the required elements, lead is present. Nickel content ranges from 15 to 16.7%, zinc - about 29%, copper - from 51 to 55%, lead - up to 1.8%. The share of other impurities is about 1%, but not more.
There are other options: MNTs 12-24, MNTs 18-27, MNTs 18-20 and others... As additives, such elements are used: iron, which imparts hardness, vanadium to impart greater density.

Often added to the composition lead, which increases the softness of the alloy, its ductility, thereby it acquires the ability to stretch, bend, twist, and it can be processed by a press. At the same time, lead reduces brittleness at low temperatures, and at high temperatures, on the contrary, increases it.

Properties
The constituent elements of nickel silver determine it chemical, physical and mechanical properties. The alloy is produced in different forms and one of them is wire, which is widely used in various industries. Metal and all its forms of release, including wire, have certain properties.

Chemical
The material has such chemical characteristics.
- It has a very high degree of corrosion resistance in any environment - both acidic and alkaline. This is especially true for acids of organic origin.
- It also shows excellent resistance to negative environmental phenomena: it does not oxidize in air and does not react with it even at a temperature of +250 degrees.
- It does not chemically interact with salts.


Mechanical
The mechanical parameters of the alloy are characterized by such indicators.
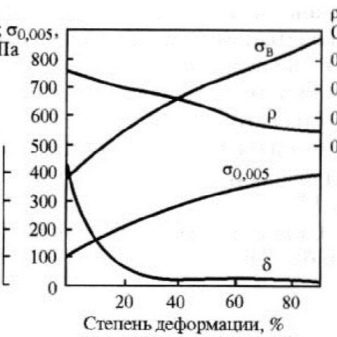
- Strength. Nickel silver is distinguished from other copper-nickel alloys by its higher strength. This is due to the fact that nickel silver is alloyed with zinc. Its strength ranges from 38 to 45 kg / sq. mm. Deformation occurs when the force is 10 kg / sq. mm.
- Elasticity... It does not have a high rate.The longitudinal elastic modulus (Young's modulus) is only 14,000 kg / sq. mm, while for ordinary steel - 20,000 kg / sq. mm.
- Plastic. This parameter is very high for an alloy. The elongation index when stretched is from 25 to 45%, and when it is narrowed - up to 32%. Good ductility is retained by the material in the cold and hot state, which provides excellent ability to various types of processing, such as embossing, broaching, stamping.
- Hardness alloy on the mineralogical 10-point Mohs scale is only 3 points. Therefore, it can easily be scratched on its surface.
- Initial melting point temperature - 1080 degrees, and the final melting occurs at 1200 degrees.
Nickel silver does not lose its appearance under the influence of the aquatic environment.
Physical
Nickel silver color depends on the percentage of nickel, and with a high percentage of nickel (up to 30%), the alloy has a whitish-silver color. With a lower amount of nickel (up to 10%), the alloy has a white color with a yellowish tinge. Nickel silver has the following physical characteristics:
- the density of the alloy is 8700 kg / cu. m;
- specific gravity - 7.5 g / cu. cm;
- low electrical conductivity due to zinc and nickel content;
- electrical resistance is characterized by a high indicator - up to 0.26 Ohm, which is 30 times higher than that of copper;
- thermal conductivity is also low - from 0.06 to 0.085 cal / cm;
- the alloy retains its shape when heated: the coefficient of linear expansion is negligible - at +100 degrees, the expansion is only about 16.6 microns.
How to distinguish from cupronickel?
Despite the external similarity, both alloys have differences. The most important difference lies in their composition.... Cupronickel does not consist of 3 main components, like nickel silver, but of 2 - copper (up to 80%), nickel (up to 18%) and minor additions in the form of iron and manganese. Cupronickel contains no zinc.
Nickel silver versus cupronickel also has a slightly greater strength and resistance to temperature extremes and external influences. Cupronickel objects in humid air quickly acquire a dark shade. Nickel silver does not darken for a long time, so it does not need frequent maintenance, like cupronickel.

These alloys also have different hardness: in cupronickel it is slightly higher, which means that the plasticity is less. Cupronickel wire cannot be bent. The difference between the 2 alloys and in cost - cupronickel is much more expensive than nickel silver.
In appearance, nickel silver does not differ from cupronickel: both alloys are similar to silver. It is possible to determine from which alloy a particular product is made only by marking. Cupronickel items are marked with 2 letters MN (copper-nickel) or "melch", as well as similar markings in a foreign language.


Nickel silver coated with silver is approved by the Russian Ministry of Health for the manufacture of cutlery, unlike cupronickel.
Application
The use of nickel silver is largely determined by its technical characteristics. Nickel, neutralizing the red color of copper during melting, gives the alloy a glossy texture with a white, whitish-blue or greenish tone. After adding lead, the gloss becomes dull and takes on a shade of gray.
Billets are made of the alloy in the form of ingots, strips, wires, rods, pipes, which are then processed in various ways. The alloy is easily exposed heating, cooling, it can be minted and forged.
Nickel silver is processed by the method cutting and forming by pressure. For the manufacture of devices or electrical equipment, the metal is polished to a shine.




Nickel silver is used in the following areas.
Radio technical industry
After removing impurities, pure nickel silver (with a content of other metals no more than 0.1%) is used for the manufacture of relays, plates for housing, springs... Due to its high anti-corrosion properties and polish purity, it is one of the main metals in the production of measuring instruments: dials and scoreboards are made from it. The divisions on the scale of such devices are usually made of nickel silver wire.


Production of cups and insignia, minting of coins
After applying gilding, a metal so similar to silver is applied for making awards. Mints of some countries use an alloy for minting coins of everyday circulation. In addition, nickel silver is often used for the release of anniversary items, limited and collectible items.


Jewelcrafting
In this area, they apply ingots, sheets and strips of alloy, but the most important thing in production is wire... It is mainly used to create various kinds of bijouterie, frames or bases for jewelry, which are then gilded.
It is considered especially popular wire stamps MSC 15-20. This brand has a melting point of about 1050 degrees, light weight and the ability to reuse without losing its original properties. It is environmentally friendly and resistant to aggressive environments. Therefore, it is her used, for example, in the manufacture of men's jewelry accessories: cufflinks, tie pins, corporate badges. In addition, wire is used to create and women's jewelry: beautiful chains and clasps, earrings and bracelets, brooches, pendants and pendants.


Medical equipment
In this area, the alloy is not so widely used, since the nickel included in the composition can cause allergy in humans... So, the alloy ceased to be used in dentistry for the manufacture of instruments and metal dentures. However, some are still made from it. separate elements of medical equipment and instruments, since the alloy tolerates disinfection well and does not react with acids and alkalis.
Food industry
Alloy makes various crockery and cutlery... And since, in direct contact with nickel silver, the products acquire a metallic taste, the products are necessarily covered with gilding or silver.
Nickel silver dishes are made according to a special standard from the brand МНЦ 15-20authorized by health authorities, by GOST 492-2006. According to the standard, the sputtering layer on cutlery should not be less than 24 microns for spoons and forks, and 18 microns for knife handles.

The crockery is characterized by high functional and decorative properties. It can be considered as a piece of jewelry and art, since valuable metals are used as a coating, and in the production process such complex finishing methods as stamping, embossing and filigree, carving and engraving, enamel are used.
The range of such items is very wide: sugar bowls and coffee pots, teapots, vases for sweets and fruits, trays, dishes of various shapes and much more. Nickel silver dishes have high hygienic and aesthetic qualities.

This metal has found application in watch industry: it is used to make watch cases, relay springs for mechanisms, as well as bracelets for watches.

Nickel silver is used and in architecture: alloy wire is used for interior design; tape is widely used for decoration of cast iron and ornamentation for exterior and interior decoration.
In artistic craft from it they make decor on the frames of wall clocks, on photo frames, and are used to create enamel and filigree. Artists and restorers often use nickel silver foil in their work.


Due to its high level of resistance to environmental influences, the alloy has found application in shipbuilding. Sheets are used in the production of parts that interact with an aqueous medium or steam: for water valves and taps, pipe fittings.


Nickel silver make pins and needles, elements of string and wind musical instruments, and fishing gear and even small arms bullets.


Care features
In accordance with its characteristics, the metal itself does not need any special care and storage conditions. However, jewelry and gilded cutlery still need to be looked after. To preserve the aesthetic appearance and extend the life of the products, you should adhere to these care rules.
- Store items in a separate box or box with a lid to protect them from direct sunlight and dust.
- In order not to damage the product, it is not recommended to try to bend it while checking its strength.
- If necessary, items made of nickel silver wire can only be cleaned with a natural bristle brush.
- On decoration signs (medals, orders), a layer of special colorless varnish should be applied to protect them from scratches.
- If the metal has darkened, then the product must be cleaned with a napkin designed specifically for the care of jewelry. Dark spots that appear can also be removed with warm vinegar, and then rinse the product well with running water. You can also use toothpaste or powder, baking soda, and dishwashing detergents to clean dishes.
- Items with a shiny, polished surface should be wiped monthly with a felt cloth, while jewelry and cutlery should be cleaned twice a month.
- Alloy jewelry should not be worn on a daily basis, and should be removed during your stay in spas and saunas.
- If the jewelry breaks down, it should be taken to a jeweler, and not tried to be repaired by yourself.




Products made of “new silver” have become part of our daily life thanks to their beautiful design, wide range of products and affordable prices.
For more information on the characteristics of nickel silver metal, see the next video.








