All about palladium

Palladium - where is it mined, what is it and what does the metal look like? All these questions arise quite often, because the name of a chemical element remains on everyone's lips thanks to jewelers and stock exchange reports. No less interesting are the methods of extraction and properties, the sample and the comparison with platinum. To find the most complete answers, you need to study as much as possible information about palladium - a metal that originated in the depths of space.


What it is?
The chemical element palladium (Palladium), designated by the Latin letters Pd, is known to every schoolchild. This noble metal belongs to the group of platinoids. In the periodic table, it is assigned atomic number 46. Palladium looks like a silver-white metal, which is extremely rare in nature. Most often it can be found in multicomponent minerals.
The palladium sample in jewelry is most often indicated by other metals. Usually gold or silver is used for these purposes. Commemorative coins of high value are made of pure 999-grade metal. The following assay marks are most often found in ingots and products: 500, 850, 900, 950 and 990.

History of appearance
The metal got its name thanks to a chain of accidents. According to the legends of Ancient Greece, Palladium was the name for the wooden face of the goddess Pallas Athena that fell from the sky - the talisman that guarded the walls of Troy. When an astronomer from Germany discovered a new celestial body (asteroid) in 1802, he named it Pallas.After some time, a chemical element was discovered, which received a similar name due to the popularization of a long-forgotten myth in Europe.


However, palladium lives up to its name... The emergence of a new metal was also not without a number of hoaxes. For example, its appearance was announced about a year before the real discovery of a chemical element. Moreover, instead of a scientifically based presentation, the sample was sent to a trader specializing in the sale of minerals in London. The bullion put up as a lot caused widespread excitement, and then it was nevertheless acquired by a chemical scientist.

Of course, the new owner of "palladium" did not buy it for the purpose of enrichment. In an effort to expose the forgery, a chemist named Chenevix made every effort to prove the artificial origin of his acquisition. It was announced that it is an alloy of mercury and platinum, synthesized according to the previously approved method of the Russian scientist Musin-Pushkin. In response to the exposure in the press, a new report appeared: the seller was offering a hefty reward to anyone who could synthesize palladium.


There was no need to pay money - the experiments were unsuccessful.
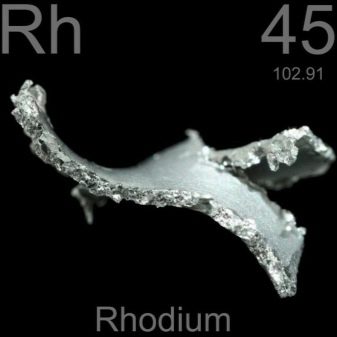
Following this, a mysterious anonym also appeared. It turned out to be William Hyde Wollaston, known by that time as one of the pioneers of UV radiation, the designer of the goniometer and refractometer, that is, a well-known and respected person... It was he who, in the course of experiments with crude platinum, was able to separate from it first palladium, and then rhodium, which are present in the composition in the form of impurities. As evidence, Wollaston provided the results of the experiments.

Interesting that for the first time it was possible to isolate palladium from ore obtained in South American lands... As a matter of fact, here, too, there was a chain of happy accidents. The original goal of Wollaston's experiments was to separate pure platinum from the impurities of mercury and gold. To do this, he used a solution of nitric and hydrochloric acids, known as aqua regia, and then precipitated the element he needed with ammonia. The results of the experiments were quite unexpected - the liquid turned pink.


In further attempts to find the reasons for the unusual coloration, the chemist used various substances. In addition to pure platinum, he managed to obtain the very same palladium - a metal lighter than mercury, an unusual light silver color. A year later, he also isolated another chemical element called rhodium from the remaining sediment.
When the story of the emergence of palladium was revealed, there was no doubt about the existence of the new metal. Scientific calculations and accurate results of chemical experiments easily confirmed Wollaston's words. Since 1805, palladium has been recognized by the world community.

Composition and properties
Palladium is a metal with the symbol Pd and an established amu. 106.42 (1) was included in the short periodic table when it was created. The pure color is silvery-white, close to silver or mercury. The metal consists of stable isotopes of the following types: 110Pd, 108Pd, 106Pd, 105Pd, 104Pd, 102Pd. Also present among the components is the isotope 107Pd, which has radioactivity with a very long half-life of substances.

Palladium isotopes can be a byproduct of a nuclear reaction. The metal itself is not radioactive at all. Its chemical and physical characteristics have the following meanings:
- boiling point - 2940 degrees;
- melts at a temperature of 1554 degrees;
- the density is 12.02 g / cm3;
- low hardness - it is a soft metal with an indicator of 373 MPa;
- does not dissolve in water;
- there is no reaction to ammonia hydrate, dilute acids and alkalis.

Palladium has high ductility and ductility and lends itself to wire drawing. Its mechanical properties can be improved by adding rhodium, ruthenium, nickel or cobalt to the alloy. The reagent in which palladium dissolves is "aqua regia".In this it is similar to platinum. Palladium itself is valuable as a chemical reagent, since it dissolves hydrogen, and active evaporation of a more volatile substance occurs in air.

Pure palladium does not magnetise. But products made from it can have such properties. Nickel and cobalt are sensitive to the magnet in alloys with palladium. When combined with gold, the latter brightens it, imparts a light silver tint even in the volume of 1-2%. Titanium, even in minimal proportions, increases its resistance to hydrochloric and sulfuric acids.
The formula of palladium oxide is PdO, oxidation occurs on contact with oxygen only when heated above 300-350 degrees. After that, a characteristic tarnished film appears on the surface. With further heating to 850 degrees and above, decomposition into pure elements occurs, the original properties are restored.


How and where is it mined?
Unlike many other platinoids, palladium occurs in its native form, this form is called allopalladium. In its pure form, it is obtained only by chemical means. Palladium is present in the sun, in iron meteorites its share reaches 7.7 g per 1 ton. The main reserves in the earth's crust are concentrated in Russia, in the Transvaal, in Colombia.

It is worth noting that the origin of palladium in the core of the planet has been proven, in this it is related to iron.
Place of Birth
The approximate volume of palladium in the bowels of the earth is estimated at 6%, in this it surpasses gold. Nevertheless, the metal still belongs to the category of rare and precious. Chemical separation involves the accompanying extraction of platinum, minerals or metals. Most often, you have to separate it from related elements.
For example, palladium platinum in the Norilsk deposit contains up to 40% palladium, and porpecite (a type of native gold mined in Brazil) - up to 10%... The main deposits and reserves of this metal are concentrated in Russia: on the Kola Peninsula, in the mountains of the Urals. There are undeveloped, mothballed objects. These are the Norilsk deposits of this valuable metal.

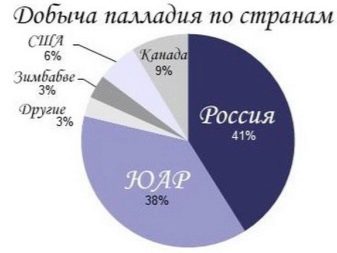
Outside the Russian Federation, palladium deposits are concentrated in Colombia, Brazil, Australia. In Canada and African countries, there are deposits of palladium-rich nickel ores. Of these, this metal is isolated - in fact, this channel is the most productive source of its production. South Africa is the second supplier of platinoids after Russia.
Production methods
Palladium is produced in a variety of ways. The pure metal can be isolated through industrial mining of ores of platinum-containing groups. Deposits of primary (primary) and loose types are used. Palladium is mined along the way, in South Africa and the Russian Federation, mainly from deposits of platinum and nickel. Pure metal is obtained in refineries, where it is isolated and concentrated into ingots or stored in powder form.


At primary deposits, only associated production of palladium is possible. On loose or secondary, it is obtained in its pure form. If the development of deposits is carried out in a quarry - open - method, ore is extracted using earth-moving equipment. Holes are drilled in the mines in the thickness of the ore layer, explosives are laid. After its detonation, the soil is refined, rises to the surface, and is sent for enrichment.

The processing of mineral rock in order to isolate valuable metals and other components is a long and laborious process. It is he who is called enrichment. On average, 1 ton of such raw materials accounts for no more than 6 g of a valuable substance. An artificial increase in the proportion of metals by making a platinum-containing concentrate helps to increase this proportion. The ore processed in this way gives up to 1.4 kg of palladium per 1 ton of raw materials.

Further production of palladium takes place at refineries. It is here that the isolation of a chemical element in its pure form is carried out.It occurs by screening out impurities through affination, after which palladium is converted into powder or fused into granules, ingots. The entire production - from industrial development of deposits to the exit of finished metal from the plant - takes about 6 weeks.

Types of alloys
All existing varieties of palladium alloys are standardized by the requirements of GOST. In the Russian Federation, the following proportions are established: 50% or 85% palladium. In Europe and North America, the base alloy has a 950 fineness, that is, it contains 95% pure palladium and 5% platinum. In Russia, the highest concentration is found - 999 g of metal per 1 kg, which is used in the minting of commemorative and commemorative coins and medals.

It is worth considering that in the amount of 1–11% palladium is included in the composition of white gold, responsible for giving the latter a light silver hue, uncharacteristic for it.
In alloys with a predominance of palladium, the following groups of metal combinations can be distinguished:
- with platinum;
- with iridium;
- with copper or cobalt and silver;
- with pure silver;
- with titanium.

The use of these compounds largely depends on the specific tasks. For example, palladium-silver alloys are most often used in jewelry... Compounds with gold or platinum are also used here. Palladium-iridium alloys are used for the manufacture of industrial semi-finished products, they are formed through deformation processes, hot and cold methods.

Comparison with other metals
The difference between metals is often looked for both in groups obtained from the same ore, and in unrelated species. This is especially often done when choosing jewelry, when you need to distinguish palladium from silver, white gold or platinum, to determine which version of the alloy is better. What to consider when comparing palladium to other metals is best discussed in more detail.
- Externally, palladium is practically indistinguishable from silver. At the same time, the difference in price between them is significant: 1 g of palladium costs as much as 100 g of silver. In this case, over time, silver darkens, but palladium does not.
- With platinum, the main difference is the specific gravity. Palladium is lighter, less dense (almost half), and dissolves in heated nitric acid. When checked with a reagent from aqua regia and a 10% concentration of potassium iodide, palladium will have a reaction, but platinum will not.
- Today, palladium is compared to gold only in the investment field. Here, this rare metal is confidently ahead of its more widely known competitor. In the presence of a palladium ligature, gold does not lose value.

All these factors should be considered when choosing a metal for investment. When buying in bullion, gold and palladium are considered the most profitable in terms of price dynamics today.
Scope of application
Precious palladium is widely used in various fields. For example, in oil production it is used as a catalyst. The metal finds a similar application in organic synthesis or hydrogenation, in working with fats. Hydrogen is purified through palladium by diffusion of materials, most often alloys of palladium and yttrium are used. In its pure form, it is used for the reversible accumulation of this substance.
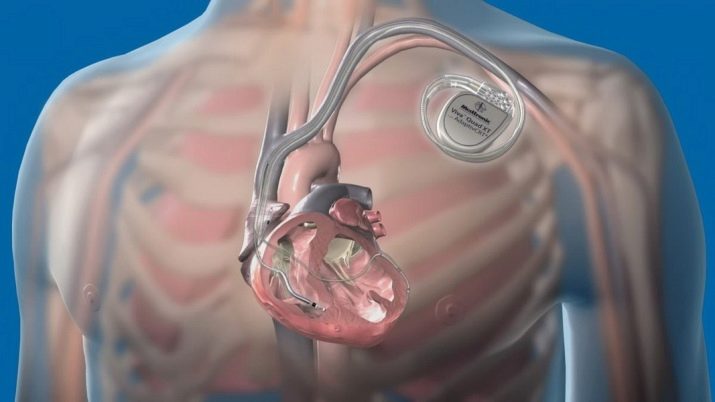
In the medical industry, palladium is used to make dentures, and it is used in pacemakers. Palladium-103 isotope is used in brachiotherapy of oncological diseases.
The use of this platinoid in electronics is highly appreciated. In the form of chloride, it is used as an activator in plating in electroplating, helping to precipitate copper. In electrical contacts, it is used as a non-oxidizable and insoluble element. This metal can be found in ceramic capacitors used in the production of television and radio equipment.

In jewelry making, palladium is best known as a ligature for producing white gold. As an independent component, palladium is alloyed with silver and platinum in a ratio of 50:50 or 85:15 parts. Wedding rings and other jewelry are appreciated, diamonds look spectacular in a palladium setting. This metal is also used as a raw material for the production of coins and medals produced in limited editions for commemorative events.

How to choose a palladium jewelry?
When choosing palladium jewelry, it is very important to pay attention to their composition. In the EU countries, where the use of nickel is prohibited, only the 950-carat metal is presented in an alloy with platinum. In Russia, there are still additives in the form of this toxic metal, which can provoke severe allergies.

If palladium is used in combination with other components, let it be silver and gold that are safe for the body.
It should be borne in mind that the modern jewelry industry represents mainly men's jewelry with this metal. Palladium cufflinks and tie clips look noble and elegant. Palladium seals and crosses look interesting.
Women's jewelry is most often made in a combined version, with enamel inserts, with precious stones. Rings, earrings, bracelets and pendants from it look interesting - you can choose options to your liking.


Care features
Palladium jewelry is still quite rare, but this metal is present in many alloys. Accordingly, it is worth considering its features when caring for metal products. The basic recommendations will remain the same as for platinum.
- Dry cleaning with soft cloths is periodically recommended.
- Wet care for heavy dirt is performed using a weak soap (alkaline) solution.
- Special wipes are available for cleaning jewelry. They can be used if you do not want to treat with liquids.
- Scratches on palladium are virtually impossible. If it is damaged, it will require a professional polishing; you will not be able to grind it by hand.
- It is not recommended to store items made of platinoids, including palladium, together with silver and gold ones. This carelessness can damage softer metals.

Considering these recommendations, it is possible to ensure maximum preservation of the attractive appearance of palladium products with different assay values.
For information on how to separate gold, palladium and platinum in the presence of copper, see the next video.








