All about precious metals

Since ancient times, people have known about the existence of precious metals. According to beliefs, they are endowed with magical properties. In addition, they are not afraid of high temperatures, the effects of acid-alkaline solutions, they shine in the sun and retain their luxurious appearance even after prolonged contact with moisture. For such properties, they were called noble.



What it is?
Metals are ferrous, non-ferrous, and also noble. The latter have always been highly appreciated by people. The more of them a person had, the more rich and influential he was considered. The high price of these metals, labor and resource intensity of mining, combined with limited reserves, became the reason why the metals of this group were called precious.
A complete list of all noble elements is specified in the Federal Law "On Precious Metals and Precious Stones" 1998. Today, this category includes eight chemical elements. They are widely demanded in various fields. These are platinum, gold, palladium and silver, and PGMs (ruthenium, radium, osmium and iridium) are also considered precious metals. Another metal, technetium, is also noble, but it has high radioactivity, therefore it is not included in the general list.
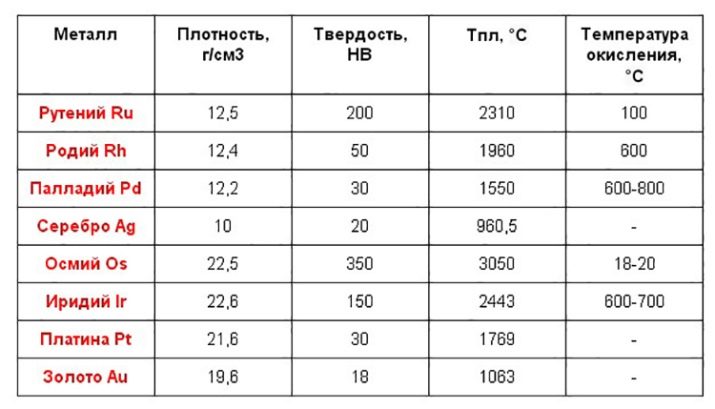
A distinctive feature of noble metals is that under the influence of unfavorable external factors, their molecular structure remains unchanged. The melting point of such elements is very high. They do not decompose in water and do not react with oxygen, therefore, do not form oxides.An alloy with such metals can be obtained exclusively through complex technical manipulations using strong chemical reagents.
The mass fraction of precious metals in the total volume of mining is small. This explains their exceptional status and increased cost.



Precious metals are non-renewable and especially valuable natural resources. None of them can be obtained by laboratory means, therefore, the appearance of these metals on Earth for scientists to this day remains a mystery. Today, there are two main hypotheses for their appearance.
- Space. According to this theory, at a certain stage of its formation, the Earth was bombarded by meteorites. It is believed that this is what led to the appearance of metals in the earth's crust. This hypothesis has a significant flaw. Scientists have found that, on average, each meteorite contains no more than 0.005% of precious metals. This does not compare with the amount that is produced in the active fields.
- Tectonic. Supporters of this version claim that precious metals appeared in the core of our planet under the influence of special conditions. And then, together with the hot lava, they were thrown onto the earth's surface. This theory is more plausible, but it does not provide answers to all questions. So, she does not explain why these fossils at some point in the development of the planet stopped forming and entering the earth's crust along with hot lava.
The topic of the emergence of precious metals is one of the most debated today. It is possible that if one day scientists are able to find the answer, then this can change the entire system of financial relationships in the country and the world.


Why was the first money made from precious metals?
Since ancient times, gold has been used as a monetary material. People have always sought to get this metal in order to then use it to purchase another desired product. To understand why this particular metal became the unit of account, you need to look into the distant past.
These days, coins are cast from aluminum, nickel and palladium. The ancient people had much less materials at their disposal: gold, silver, copper, lead, tin, and also iron. Of these, only two did not oxidize on contact with air and water and were considered noble. These metals themselves were already of high value. And they also possessed the characteristics necessary for the manufacture of a universal monetary equivalent.


Let's consider these characteristics in more detail.
- Uniformity. A pair of pieces of the same precious metal, having the same weight, have the same value. That is why such a metal is optimal for expressing the price of products. All its copies are identical, their difference lies only in the mass.
- Divisibility. Unlike other monetary equivalents accepted in antiquity, for example, cattle and furs, precious metals can be divided into several parts without losing their value. This is very important for any currency that must serve the exchange of goods of very different values.
- Wastelessness. This characteristic follows from the previous one. Dividing a piece of precious metal does not generate waste, more or less valuable parts, and the total cost remains unchanged.
- Mobility. The coins are easy to use. They are lightweight and easy to carry. Thus, even small amounts of gold passing from hand to hand have a rather high cost, so they can serve the circulation of large volumes of relatively cheap items.
- Persistence. Noble metals do not deteriorate, rust does not eat them, rot does not appear on them. Accordingly, as they are stored, they do not lose their intrinsic value.
Finally, gold, silver and other precious metals have always been a universal store of value, the so-called treasure.Throughout history, regardless of the political regime, social situation, changes in state borders and movement from one country to another, these metals were and remain valuable.
All these characteristics led to the fact that for many centuries the function of monetary materials was firmly entrenched for precious metals.


Species overview
Noble metals got their definition due to special physical and chemical properties. Depending on the type of metal, these parameters can be felt to a greater or lesser extent. But in any case, they will be unique.

Rhodium
This metal belongs to the group of platinum metals. It is light blue in color and belongs to the light metal category. It is distinguished by a high degree of fragility and, at the same time, exceptional hardness. It is in demand due to its unique reflective characteristics. This metal is resistant to aggressive chemicals, it can be oxidized exclusively with heated sulfuric acid. The melting process takes place under a thermal effect of 2000 degrees.


Platinum
Platinum was first discovered in the mines of America, and because of its whitish luster it was formerly called "silver". Only in the middle of the 18th century, the metal received the status of precious, and in a very short time its price overtook silver and gold. The material is plastic, refractory, lends itself well to forging, thanks to which it is very popular with jewelers.
Moreover, platinum is harder than gold, it is resistant to acid-base effects. Does not oxidize.


Gold
Gold is characterized by good malleability and exceptional ductility. Unlike platinum, it melts at lower temperatures. It is invulnerable to the effects of acids, alkalis and caustic salts; it can react only with aqua regia. Pure gold has the typical luster and rich yellow hue, but is very rare in the natural environment. Mostly miners extract greenish ore.


Osmium
White noble metal. Differs in increased resistance to aggressive chemical and physical factors. The melting point corresponds to 2700 degrees.


Iridium
Iridium is classified as a heavy metal. It is the most dense and strongest. Does not dissolve in caustic acids and alkalis. Melts when heated at 2450 degrees. Has a grayish-white tint.


Ruthenium
In terms of visual characteristics, ruthenium can be confused with platinum, and in terms of fusibility, this metal has the same characteristics as iridium. It is distinguished by its density and exceptional strength. It can form water-soluble cakes when exposed to oxidants, alkalis and elevated temperatures.


Palladium
Soft metal with a white color with a pronounced silvery sheen. Melting temperature - 1550 degrees. When heated to 850 degrees, it begins to form oxides, but with a subsequent increase in heating it becomes pure again.


Silver
Among all precious metals, silver has a relatively low melting temperature - only 960 degrees, as well as a minimum density. Nevertheless, this material hardly interacts with acids and serves as a reliable heat and electrical conductor.
However, under the influence of hydrogen sulfide, it darkens in the atmospheric air.


Features of mining and production
Precious metals are non-renewable elements. Their placers are almost never found on the surface of our planet. Today, gold mines are more like underground reservoirs, in which the mined ore is first transformed into a solution, and then filtered and re-processed.
Gold and silver are today becoming a by-product of ore extraction in the underlying mining industry. Such mines on an industrial scale are not developed as independent ones.This is due to the fact that the presence of noble elements in the earth's crust is minimal, so their extraction will be unprofitable.


The ore mined by the miners is unsuitable for use without further purification and processing. Let's take a closer look at the process of preparing a precious metal using the example of gold.
- The first stage of processing is the cyanidation of the ore. This technique involves exposing the ore to cyanide and then filtering the gold sediment. The result is a concentrate.
- Schlich goes to the experimental laboratory, where physical and chemical research and testing for radioactivity are carried out.
- After that, the concentrate is sent for refining. - the so-called cleaning. Technically, this process is liquefaction, then filtering and subsequent recovery of the feedstock. The difference is that there are no impurities in the refined metal.
- The gold alloys obtained as a result of such processing are used for casting.



Analysis
The precious metal analyzer aims to answer two basic questions:
- what raw materials are in front of us: pure precious metal or an alloy with an insignificant content of a noble element;
- what is the percentage of precious metal in the alloyed mass presented for analysis.

The first sample is qualitative, the second gives a quantitative result. They are performed in strict sequence, one after the other. After conducting a quality test, which establishes that the alloy really contains a precious metal, you can proceed to determining its amount. If, when examining the analyzed sample by interaction with assay acid, nothing remains, then it is a base metal.
The results established during the examination were reflected in the samples. This is a numerical marking, it shows the percentage of precious metal in the presented alloy.
However, it should be borne in mind that the test in Russia is applied not to all alloys, but only to those in which the concentration of the noble element is more than 30%.

Applications
Precious metals are widely used in various fields. Here are just a few of them.
Electrical engineering
Unique physical and technical characteristics in tandem with chemical and biological inertness make it possible to create effective protection of electrical contacts from burning and oxidation. This makes the metal safe and practical for electrical applications.
It is no coincidence that alloys of most precious metals are in widespread demand in the manufacture of high-precision instruments.
Silver salts (chlorides and bromides) are used to create photosensitive elements. Solders made of noble metals are in demand in the creation of electrical devices, which are subject to increased reliability requirements. The rarest elements are used to create thermocouples and other heating elements.
Jewelcrafting
From time immemorial, precious metals have been in demand in the jewelry industry. They are used to create exclusive chains, earrings, bracelets, rings, pendants, crosses, as well as spectacle frames, expensive cigarette cases and many other products. Jewelers appreciate the color, exquisite luster of metals and their unique properties.
Precious metals do not react with human skin, so they do not lead to skin diseases and allergic reactions. It is allowed to use precious metals as a sputtering layer for jewelry made from cheap metals. Such jewelry delights its owners for many years and is often inherited from generation to generation.


Chemistry
The resistance of precious metals to acid-base compositions, as well as catalytic parameters, make their use in the chemical industry topical. They are used to create equipment for aggressive compounds. Many of these metals have found use as catalysts in the production of gasoline.

Automotive
Catalysts are also used to create exhaust gas devices. That is why precious metals are in demand in the manufacture of auto parts. They allow you to quickly and reliably neutralize toxic chemical compounds. Most often, palladium and rhodium are taken for these purposes.

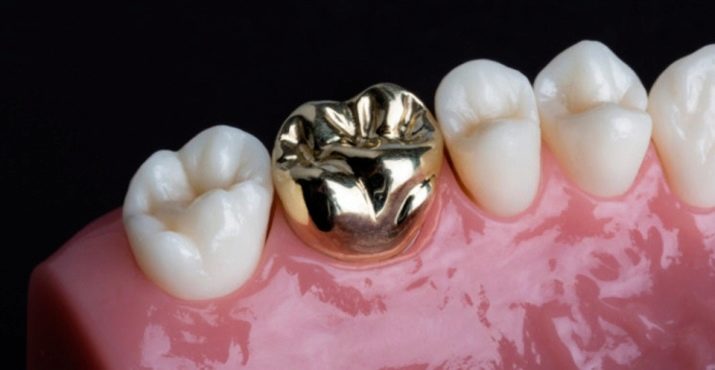
Medicine
Biological and chemical inertness allows using precious metals in the production of surgical instruments and all kinds of parts for medical equipment. Many metals are in demand in prosthetics and dentistry. A number of compounds have become widespread in the manufacture of medicines as a constituent component.
Space science
Precious alloys are relevant in the construction of aircraft and spacecraft, since only they are able to ensure the maximum reliability and safety of these systems. Only a noble metal can cope with the loads that a space station can experience in orbit.

Glass industry
Precious metals have also found their application in the manufacture of glass. Very often glass melting tanks are made of them.

Banking sector
Also, one cannot fail to mention the role of precious metals as an exchangeable monetary measure. Gold and silver were used in ancient times to make coins, although nowadays silver has already lost its function in this circulation. And yet, investment bars are still cast from gold and platinum to this day.
This allows everyone to invest free funds with high profit. As practice shows, the traditional currency depreciates over time, while gold bars invariably remain in value.
Nowadays, anyone can invest their savings in precious metals of the highest standard.

Many banking and financial organizations even offer depositors to open special metal accounts. This is a profitable investment, since in the long run the owners of such bars can get serious profits. Metal accounts have only one drawback - the absence of a deposit insurance system, which can entail a considerable risk in the event that the bank goes bankrupt.








