All about silver

Silver is a fairly common metal. In jewelry, it is often combined with transparent artificial stones. The flexibility and ductility of silver makes it possible to make unusual and intricate items from it. The metal is quite diverse and is used in many areas of life.



What it is?
Silver is a precious metal and chemical element. Argentum - 47 element in the periodic table. Silver ore looks like a common rock. The metal is soft, ductile, has the highest electrical and thermal conductivity. It should be noted that the chemical activity is weak.
The precious metal is used in many fields. So, it will come in handy in the jewelry, electronic, photography and film industries. Due to its bactericidal properties, the mineral is also used in medicine.
It is worth noting that the nugget contains many minerals. Silver ore is rare, so the metal is mined from complex polymetallic ores.



Discovery history
Silver has valuable properties, so people knew about it long before the beginning of our era. It is impossible to establish an exact date. The first mentions are found among the Egyptians, they made dishes from silver. Jewelry made of this metal dates back to 5000-3400 BC. NS. Previously, people only knew about native silver.
Numerous experiments allowed alchemists to discover the presence of metal in various compounds. Then it made sense to define silver as a chemical element. Then scientists became interested in the healing and bactericidal properties of the material. In Russia, the metal was first smelted by Lavrenty Neigart in 1687.

Composition and properties
Silver has a white metallic luster. The hardness of the metal is 25 kgf / mm². Thanks to this property, the material is so strong and durable. The density of Argentum is 10.5 grams per cm3. The melting point is quite high, reaching 962 ° С.
Metal is able to withstand heavy loads. This allows it to be used as a material for many assemblies, even space rockets and submarines. Silver reflects light well, which expands the scope of use. And also the metal conducts heat well.
Leaving a silver spoon in a cup of hot tea can burn you.


Chemical properties of the substance are no less interesting. For example, a metal can dissolve in mercury and turn into an amalgam. The chemical element does not react with oxygen, carbon, hydrogen, silicon and nitrogen. However, silver interacts with other substances that are important in everyday life.
- Sensitivity to hydrogen sulfide. Even a small amount leads to the formation of silver sulfide. Visually, a black bloom is visible on the product. Sulfur compounds surround people all over the place. The substance is found in food, building materials, and even in human sweat.
- Reacts with halogens, especially iodine. Contact of silver with this substance should be avoided.
- When heated, Argentum adsorbs gases... We are talking about oxygen, argon, hydrogen and the like. Liquid metal absorbs many times more O2 than solid metal. When solidified, you can see how oxygen breaks through the upper crust. It looks like a small silver volcano.

Mining
Any metal must initially be taken from somewhere. Being in nature, in mines, is the main source of silver mining. Moreover, 2/3 of the total amount is a by-product in the production of nickel, copper, lead and zinc. And silver is mined through recycling. However, the efficiency of the process is low due to its complexity.


What happens?
Everyone knows that gold can be pink and white, not just red. That's jewelry silver is colored. Due to this, the metal looks different in products. True, exotic colors are not in high demand among buyers. Native silver is quite noble and attractive.
The metal is divided into types depending on the additional materials in the alloy composition... Additives directly affect important properties.
So, some types of silver are suitable for weaving chains and bracelets. Sometimes special sprays play a purely decorative role and even complicate the care of the product.

Sterling
This type of Argentum is defined as a standard. It contains 92.5% of this metal and only 7.5% of copper. The additive is needed to increase the strength of the finished product. The alloy has a natural white tint.

Matt
The shine can be removed by heat treatment in sulfuric or hydrochloric acid.... Matte silver is used to create jewelry.
This look without shine looks stylish and quite expensive. The decorative properties are of particular interest to matt silver.

Filigree
Such a metal is different breakdown 960. In jewelry, silver is used for delicate braided patterns.
Interestingly, only 1 gram of metal needs to be taken to make 2 km of wire. Such decorations require careful use.

Rhodium
Such silver is considered one of the most expensive... A thin layer of platinum group metal allows for increased wear resistance and elasticity. This coating protects the jewelry from oxidation.
Jewelry made from such silver can be cleaned much less frequently than others.

Blackened
Such an interesting look was invented in Russia back in the 17th century. Depressions in the product are filled with an alloy of copper, lead and silver sulfides. As a result, the treated areas turn black, while all the ridges remain white.
The products should be cleaned very carefully so as not to destroy the dusting.

Oxidized
Blackened silver subspecies. Silver oxide film is used to create visual effects. In this case, all cavities can be blue, gray, purple or black.
Jewelry requires special care when cleaning.

Gold plated
Looks like gold, but costs less... Products should be used with care to avoid rubbing off the yellow powder.
Do not allow gold plated silver to come into contact with chemicals and even water. Any aggressive mechanical action is prohibited.

Application
The area of use of silver is so large that it is difficult to list everything at once. Moreover, the metal is often used in small quantities. Physical and chemical properties make Argentum attractive for jewelry making... The same features make it possible to use silver in the manufacture of technical devices in medicine.
For jewelry and cutlery do not use pure metal. Its plasticity and flexibility simply do not allow it to do so. Copper and the like are added to the alloy to improve deformation resistance. The more additional material, the less flexibility.

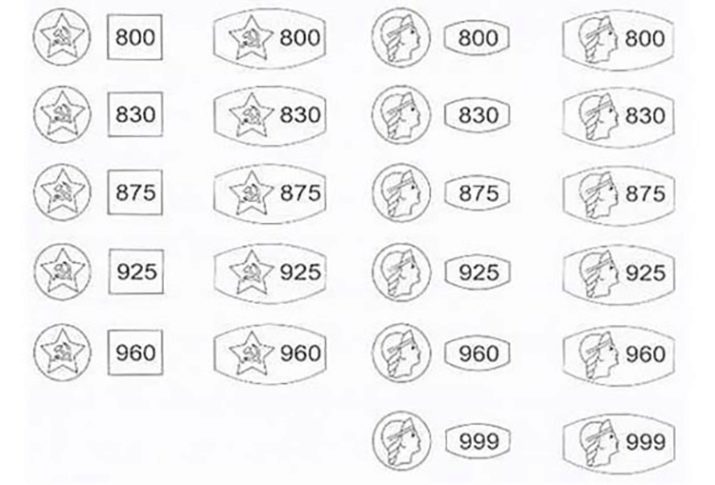
It is worth noting that in any field, an alloy of silver is used, not a pure material. A sample is used as a measure. The three-digit number shows how much silver is in 1 kg of a particular alloy.
Several official sample variants are officially recognized in Russia.
- 720. The yellowish alloy is used to make springs, needles and parts that are subject to high stress.
- 800... Metal with a yellowish tint is used to create jewelry and cutlery.
- 830... The scope of application is the same as in the previous version.
- 875... Used in the jewelry industry. Jewelry is often gilded.
- 916. Previously used to make cutlery. Now it is not used in jewelry at all.
- 925... Standard Sterling Silver. Especially appreciated when creating jewelry due to the fact that it practically does not oxidize. It can be used in the manufacture of cutlery.
- 960... The alloy is used to create products with relief compositions. Jewelry is easily deformed.
- 999... Raw materials for making coins and ingots. It is also used for electrical equipment, ionizers and air purifiers. This metal is used in the manufacture of mirrors with maximum precision. Pure Argentum can be found in medicines.
The scope of application is not limited to this list. Silver can be used in the food industry. Apparatus for squeezing juice and making other drinks are made from it. Bars and powder can be found in batteries.
In general, it is in the industry that the largest amount of silver is used. Especially in electrical engineering. Silver chloride is used as a coating for radar surfaces. So, metal can be found in production:
- radio electronic components;
- lead-acid batteries;
- infrared optics;
- gas mask filters;
- some detonators.

Interesting Facts
- Recent years have brought many interesting discoveries. All of them allowed discovering new possibilities of using silver in jewelry, industry and medicine. There is a Silver Institute in the world. It is this authority that informs about the prospects for the use of metal.
- Scientists from California have developed a new method of using silver for the manufacture of solid metal. The substance will combine the best qualities of this group of elements with glass. The resulting material can be used to make medical implants. Scientists are confident that the new product is significantly better than the existing counterparts.
- The presence of silver reduces the risk of infection. This is due to its unique antibacterial properties. Of course, metal-glass has not been sufficiently researched yet. But the very fact of creating such a material is very curious.
- Unusual nanoparticles are made from silver. They are used as sensors to detect disease-causing bacteria. Respiratory mask with silver can kill many microorganisms. Society also owes this to science.

For even more interesting facts about silver, see the next video.








