Silver contacts: how to extract?

Many people still keep radio components from the times of the USSR at home. They contain silver contacts that can be removed at home. To get a precious metal, you will need to study the procedure and prepare special items.


Peculiarities
Electrical contacts may contain pure silver. There are several types of connections.
- Those that do not magnetize... The silver content in this group is the highest.
- Magnetizable... They have the least amount of pure metals.
- Copper... Solders are a copper plate that is covered with a small layer of silver.
Silver contacts are used in low power devices. Pure metal can be obtained from:
- electromagnetic starters;
- electric machines;
- relay;
- temperature sensors;
- silver-zinc rechargeable batteries.


Care rules
Silver contacts need to be monitored. Care consists in periodically checking their condition. If they are dirty, you should resort to cleaning with a suede cloth or cloth.... It will need to be slightly moistened with gasoline. Can be brushed with a stiff hair brush.
Sagging is removed with a file.

How do I separate the silver?
The extraction of pure silver from contact alloys has long been practiced. To extract pure metal, you will need a special purification technique called refining. This procedure is divided into several steps, the result of which is always the allocation of silver.
Although refining is an industrial procedure, you can also desolder precious metals yourself at home. There are different ways.

Cupellation
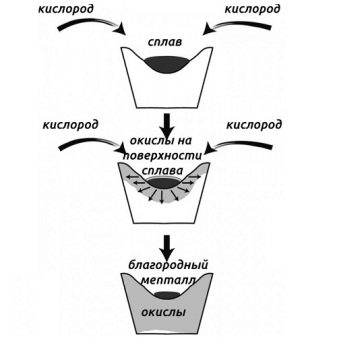
Silver can be isolated from low-grade alloys by cupellation.This method is based on the ability of molten silver with lead to begin to oxidize in air. This contact will separate the metal. For work, you will need a special oven equipped with a bowl-shaped crucible. The bowl should be with a marl coating based on porous limestone clay.
The process is divided into several stages:
- the oven is warming up;
- then an assay crucible is placed in it, in which technical silver and lead already lie;
- heat up the crucible until complete melting occurs;
- after that, air masses are launched into the furnace.
A thermal reaction is included in the process. When it is over, you can take out the crucible and pour its contents into molds.
After cooling down, the alloy takes on a rainbow color scheme. Similar reports that, in addition to silver, it contains other precious metals.

Electrolytic method
To extract the metal in this way, you need special plastic or sandstone cells with silver nitrate. The content of the precious metal in the liquid is at least 50 g / liter. Contaminated silver will be used as the anode. The cathode in this situation is stainless steel, cut into strips of small thickness.
The anodes are placed in textile bags. These bags will then collect contaminated silver particles that have not been able to dissolve. Silver macrocrystals will be deposited in the cathodes. They will rise towards the opposite pole until short-circuited. To prevent a short circuit from happening, the branches of the crystals are subjected to a break during mixing of the solution.... Such crystals settle on their own at the bottom, from where they are removed. Ingots can be smelted directly from these components.

Chemical
Extraction of silver metal from solutions or salt will be carried out using a chemical method. As a result, black silver sulfate will be obtained. To use this method, you will need the obligatory use of sodium sulfate.... The whole procedure will last until the silver sulfate stops being released.
If this method is chosen, the precious metal is obtained only in the form of chloride and only after the addition of a certain substance has been made. You can use ammonium chloride or table salt... As a result, a liquid will be obtained, which must be settled until it is divided into two parts: unclear and clean.
Advice! If salt is added in the future and the solution does not become cloudy, it means that all the precious metal has settled to the bottom.

If you need to work with chloride, there are two methods:
- casting - using alkali metal carbonate;
- with a solution - it will be necessary to bring the sample to the highest values.
Each of the methods described can be carried out independently at home. For work, you will need special substances and tools. The simplest method for people who have no experience in refining is electrolytic.
It is divided into three stages:
- initially, a precious metal dissolves in nitric acid;
- then it is cemented;
- the final stage is fusion.


In order to carry out the first stage, you will need to take a nitric acid solution, the concentration of which is 68.8%. Along with this, you need to prepare a glass container and a quartz rod. When working with acid, you need to follow safety precautions and be in a well-ventilated area. The best option is outdoor interaction.... The skin of the hands should be protected with gloves, and the eyes should be covered with glasses. Advice! It should be remembered that acid is added to water. It is prohibited to pour water into acid.
To dilute nitric acid, you will need to take deionized water and pure acid. It is necessary to observe the ratio of 1: 1. The formed liquid is stirred with a quartz rod.Then the acid is poured into glass containers designed for reagents. Silver nitrate must be produced in such a way that it is sufficient for the entire procedure. The approximate concentration is 50 grams per liter. Now you need to dissolve the silver in the liquid. Usually, during this procedure, NO2 is released, after which the solution acquires a blue tint. Immediately you need to be prepared for the fact that the procedure takes a lot of time. Entire precious metal will dissolve only after 10 hours... The resulting solution is closed in a chemical jar.


The next stage is silver cementation. It will require separating metallic silver from nitrate, which contains copper. To do this, add copper to the silver nitrate. You can use old plumbing pipes that have been previously cleaned to a shine. When copper is added, the reaction is accelerated.... As a result, cement silver is formed on the tubes, which looks like a powder. So that the reaction rate does not decrease, it will be necessary to periodically remove the cement from the pipes into the solution.
The whole process becomes possible due to the fact that the tubes transfer copper to silver nitrate, which gradually leads to complete dissolution. If the tubes are gone, they need to be replaced. As the silver is displaced, the reaction begins to slow down. For this reason, you can not follow the process and leave it for a couple of days.
All that is needed is to observe the presence of copper and to ensure that no unnecessary objects are present in the solution.


It is possible to determine that the reaction is complete by the cooled solution, in which there are no signs of an active process: at the top, the liquid will acquire a blue and pure color, and cement will be located below. When the reaction is over, the cement will need to be filtered.
For work you need to prepare:
- funnel;
- coffee filters;
- the container where the raw material is removed.
Advice! It must be remembered that filtration is carried out repeatedly. At least five treatments are required.



This approach will remove all copper nitrate particles from the cement. At the end of filtration, you need to evaporate excess moisture or wait until it is removed naturally.
In the next video, you will find yourself cleaning technical silver at home.








